Mechanism of alkane H/D exchange over zeolite H-ZSM-5 at low temperature: a combined computational and experimental study†
Abstract
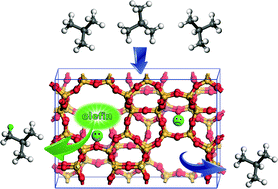
Theoretical calculations have provided fundamental insights into the possible pathways for the H/D exchange of isobutane with H-ZSM-5 zeolite at room temperature. It is theoretically demonstrated that neither the direct exchange mechanism nor the indirect bimolecular hydride transfer mechanism is an efficient route for isobutane activation due to high activation barriers, which possibly prohibit H/D exchange at low temperatures. It is revealed that a trace of olefin impurities can considerably accelerate the formation of an alkoxyl intermediate which is involved in the bimolecular hydride transfer mechanism. Once the alkoxyl intermediate is generated from the olefin impurities, the catalytic cycle is self-sustaining. On the other hand, the theoretical calculations also illustrate that the extra-framework aluminum (EFAl) species in the dealuminated zeolite has no obvious promotional effect on the H/D exchange. Furthermore, our calculations are also consistent with the experimental results.

 Please wait while we load your content...
Please wait while we load your content...