High-temperature water gas shift reaction on Ni–Cu/CeO2 catalysts: effect of ceria nanocrystal size on carboxylate formation†
Abstract
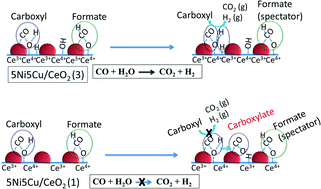
Thermally stable CeO2 nanospheres of various controllable sizes were successfully synthesized via a PVP-assisted hydrothermal method to study the effect of ceria crystal size in high-temperature water gas shift reaction. The intrinsic properties of ceria crystal size effect was explored using X-ray Diffraction (XRD), Field Emission Scanning Electron Microscope (FESEM), Brunauer, Emmett and Teller Surface area (BET), X-ray Photon Spectroscopy (XPS), Carbon monoxide-Temperature Programmed Reduction-Mass Spectrometry (CO-TPR-MS), and in situ Diffuse Reflectance Infra-red Fourier Transform Spectroscopy (DRIFTS) techniques. The XRD, FESEM and BET results indicate that the ceria with the largest particle size and the smallest crystal size of 12 nm shows a high specific surface area of 50m2 g−1 after calcination at 700 °C. After impregnation, high metal dispersion (15%) and a high amount of surface lattice oxygen are observed on the Ni–Cu bimetallic catalyst supported on ceria with the largest particle size. This Ni–Cu/CeO2 catalyst presents high reaction rates with low apparent activation energy as compared to other Ni–Cu/CeO2 catalysts, revealing the important effect of ceria crystal and Ni–Cu alloy sizes. A further study shows that the high amount of carboxylate species on the 5Ni5Cu/CeO2 catalyst with the biggest ceria crystal size could be the inhibitor or the real intermediate species. In addition, the reaction mechanism strongly depends on the Ni–Cu surface composition.

 Please wait while we load your content...
Please wait while we load your content...