The cascade synthesis of α,β-unsaturated ketones via oxidative C–C coupling of ketones and primary alcohols over a ceria catalyst†
Abstract
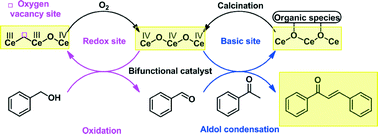
We herein report the oxidative C–C coupling of ketones and primary alcohols to produce α,β-unsaturated ketones in the absence of base additives. This cascade synthetic reaction was conducted at 150 °C in 12 h using a heterogeneous CeO2 catalyst. The conversion of acetophenone reached 74% with 89% selectivity to chalcone. A correlation between the CeO2 crystal plane and catalytic performance is established as the catalytic activities decrease in the sequence of (110) > (111) > (100). Characterization using Raman spectroscopy, CO2 temperature-programmed desorption (CO2-TPD), and in situ active site-capping tests has shown that the unusual catalysis of the CeO2 catalyst is attributed to the coexistence of basic and redox active sites. These sites synergistically catalyze the oxidation of alcohols to aldehydes and the aldol condensation to ketones. Moreover, the CeO2 catalyst can be reused several times after calcination to remove the surface-adsorbed substances.

 Please wait while we load your content...
Please wait while we load your content...