Silica/MAO/(n-BuCp)2ZrCl2 catalyst: effect of support dehydroxylation temperature on the grafting of MAO and ethylene polymerization†
Abstract
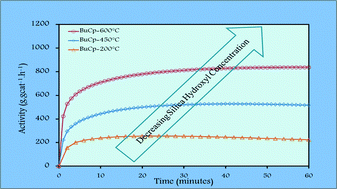
The current study aims to explore the link between the role of silica dehydroxylation temperature and the catalytic performance of an (n-BuCp)2ZrCl2 metallocene precursor supported on silica impregnated with methylaluminoxane (MAO) in ethylene homopolymerization. Diffuse Reflectance Fourier Transform Infrared Spectroscopy (FT-IR-DRIFT) and Solid State Nuclear Magnetic Resonance (SS NMR) were used to study the generation of different Al species on the surface of silica impregnated with MAO after calcination at temperatures of 200°, 450° and 600 °C. It was found that Si–CH3 bonds can be formed when silica dehydroxylation temperature is ≥450 °C, and that three different types of aluminum species are generated on silica surface, the nature of which does not change significantly with the silica dehydroxylation temperature. After grafting a metallocene precursor on the MAO-treated surfaces, increases in the zirconium loading, and in the intrinsic and average catalytic activities were observed with increasing silica dehydroxylation temperature. However, the molecular and physical properties of the high density polyethylene produced by using these catalysts were observed to be independent of the silica dehydroxylation temperature. These results indicated that the number of active sites on the surface of the catalyst increased with increasing dehydroxylation temperature, but the nature of the active sites does not.

 Please wait while we load your content...
Please wait while we load your content...