Highly selective oxidation of cyclohexene to 2-cyclohexene-1-one in water using molecular oxygen over Fe–Co–g-C3N4†
Abstract
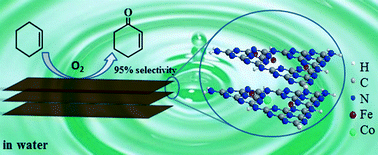
Efficient and greener oxidation of cyclohexene to 2-cyclohexene-1-one is an interesting topic. In this work, we prepared a series of Fe–Co doped graphitic carbon nitride (Fe–Co–g-C3N4) catalysts through simple impregnation and calcination methods. The catalysts were characterized by different techniques, such as transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), nitrogen adsorption–desorption measurement, powder X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The selective oxidation of cyclohexene to 2-cyclohexene-1-one was carried out in different solvents over the catalysts using molecular oxygen as an oxidant. The influence of supports, solvents, Fe/Co molar ratio in the catalysts, pressure of oxygen, reaction temperature and time of the reaction was investigated. It was revealed that the bimetallic Fe–Co–g-C3N4 catalysts were very efficient for the reaction. More interestingly, the selectivity of the reaction in water was much higher than that in other solvents. Under optimized conditions, the selectivity to 2-cyclohexene-1-one could reach 95% at a cyclohexene conversion of 36%. The Fe–Co–g-C3N4 catalyst could be reused at least four times without obvious loss of efficiency.
- This article is part of the themed collection: 2016 most accessed Catalysis Science and Technology articles

 Please wait while we load your content...
Please wait while we load your content...