Synthesis of cyclic monothiocarbonates via the coupling reaction of carbonyl sulfide (COS) with epoxides†
Abstract
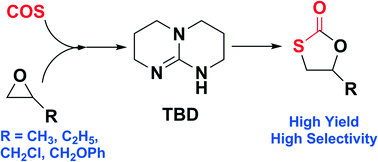
Two guanidine bases were used as organocatalysts for the synthesis of cyclic monothiocarbonates via the coupling reaction of carbonyl sulfide (COS) and epoxides. The systems proved to be efficient single-component, metal-free catalysts for the reaction of simple (propylene oxide, 1,3-butene oxide) or activated epoxides (epichlorohydrin, glycidyl phenyl ether) with COS under solvent-free and mild reaction conditions to selectively afford the corresponding cyclic monothiocarbonates. The yield of this reaction is generally high, thereby providing ready means for pure product isolation.


 Please wait while we load your content...
Please wait while we load your content...