Surface Lewis acid-promoted copper-based nanocatalysts for highly efficient and chemoselective hydrogenation of citral to unsaturated allylic alcohols†
Abstract
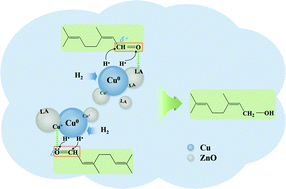
Chemoselective hydrogenation of α,β-unsaturated aldehydes or ketones to unsaturated alcohols (UAs) is one of the key processes for the production of various important intermediate chemicals. In the present work, well-dispersed ZnO-promoted supported copper nanocatalysts were generated from Cu–Zn–Al layered double hydroxide (CuZnAl-LDH) precursors for liquid-phase chemoselective hydrogenation of citral to allylic alcohols (geraniol and nerol isomers). A series of characterizations including XRD, TEM, STEM, XPS, H2-TPR, and Py-IR demonstrated that the microstructure and catalytic performance of as-formed Cu-based nanocatalysts were significantly affected by the incorporation of Zn into catalyst precursors. It was found that the addition of more ZnO to catalysts could result in better metal dispersion and an increase in the surface Cu+/(Cu+ + Cu0) ratio and surface Lewis acid sites. In liquid-phase chemoselective hydrogenation of citral, a high selectivity toward allylic alcohols (>75%) at complete citral conversion was achieved successfully on as-formed non-noble-metal Cu-based nanocatalysts with a Cu/Zn molar ratio of 2 : 1 under mild reaction conditions (e.g. 80 °C, 1.0 MPa). The high efficiency of the catalysts was attributed mainly to both the synergism between Cu0 and Cu+ species and the promotion of surface Lewis acid sites, thereby improving the dissociation of hydrogen and facilitating the adsorption of the citral molecule and the following activation of the carbonyl group during the citral hydrogenation.

 Please wait while we load your content...
Please wait while we load your content...