Conversion of syngas-derived C2+ mixed oxygenates to C3–C5 olefins over ZnxZryOz mixed oxide catalysts
Abstract
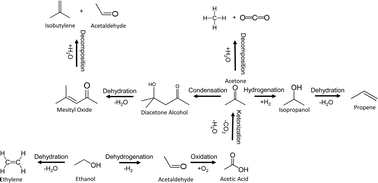
In this study we report on a ZnxZryOz mixed oxide type catalyst capable of converting a syngas-derived C2+ mixed oxygenate feedstock to isobutene-rich olefins. Aqueous model feed comprising of ethanol, acetaldehyde, acetic acid, ethyl acetate, methanol, and propanol was used as representative liquid product derived from a Rh-based mixed oxygenate synthesis catalyst. Greater than 50% carbon yield to C3–C5 mixed olefins was demonstrated when operating at 400–450 °C and 1 atm. In order to rationalize formation of the products observed feed components were individually evaluated. Major constituents of the feed mixture (ethanol, acetaldehyde, acetic acid, and ethyl acetate) were found to produce isobutene-rich olefins. C–C coupling was also demonstrated for propanol feedstock – a minor constituent of the mixed oxygenate feed – producing branched C6 olefins, revealing scalability to alcohols higher than ethanol following an analogous reaction pathway. Using ethanol and propanol feed mixtures, cross-coupling reactions produced mixtures of C4, C5, and C6 branched olefins. The presence of H2 in the feed was found to facilitate hydrogenation of the ketone intermediates, thus producing straight chain olefins as byproducts. While activity loss from coking is observed complete catalyst regeneration is achieved by employing mild oxidation. For conversion of the mixed oxygenate feed a Zr/Zn ratio of 2.5 and a reaction temperature of 450 °C provides the best balance of stability, activity, and selectivity. X-ray diffraction and scanning transmission electron microscopy analysis reveals the presence of primarily cubic phase ZrO2 and a minor amount of the monoclinic phase, with ZnO being highly dispersed in the lattice. The presence of ZnO appears to stabilize the cubic phase resulting in less monoclinic phase as the ZnO concentration increases. Infrared spectroscopy shows the mixed oxide acid sites are characterized as primarily Lewis type acidity. The direct relationship between isobutene production and the ratio of basic/acidic sites was demonstrated. An optimized balance of active sites for isobutene production from acetone was obtained with a basic/acidic site ratio of ∼2. This technology for the conversion of aqueous mixtures of C2+ mixed oxygenates provides significant advantages over other presently studied catalysts in that its unique properties permit the utilization of a variety of feeds in a consistently selective manner.

 Please wait while we load your content...
Please wait while we load your content...