Oxidative coupling of anilines to azobenzenes using heterogeneous manganese oxide catalysts†
Abstract
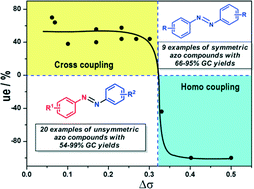
We herein report the transition metal oxide-catalyzed synthesis of azobenzenes through the oxidative coupling of anilines. An octahedral molecular sieve of manganese oxide, OMS-2, exhibited the best activity and selectivity. Nine examples of symmetric azobenzenes and twenty unsymmetric ones were synthesized with 62–99% conversion and 64–99% selectivity. In the aniline cross-coupling reactions, the difference of the Hammett constants of two substituted groups (Δσ) determines the selectivity to unsymmetric azobenzenes, which are the major products at Δσ < 0.32. In-depth studies reveal that the surface defect sites of the mixed-valence manganese oxide play a key role in facilitating electron transfer and activating molecular oxygen. The single-electron transfer (SET) reaction mechanism is proposed based on electron paramagnetic resonance and X-ray powder diffraction characterization.

 Please wait while we load your content...
Please wait while we load your content...