Experimental and theoretical studies on compositions, structures, and IR and NMR spectra of functionalized protic ionic liquids†
Abstract
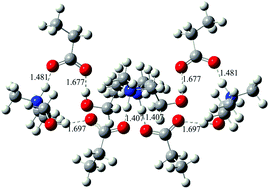
The compositions and structures of amine-based functionalized protic ionic liquids (PILs), namely N,N-dimethyl(cyanoethyl)ammonium propionate (DMCEAP) and N,N-dimethyl(hydroxyethyl)ammonium propionate (DMEOAP) have been investigated systematically by IR and 1H NMR spectroscopy and density functional theory (DFT) calculations. Analysis of the IR spectra suggests that both DMCEAP and DMEOAP are composed of neutral and ionized species in the liquid phase, the former one mainly existing in the state of precursor molecules, and the latter mainly as ion-pairs. The ratio of precursor molecules to ion-pairs in the liquid phase depends on the types of precursors, especially the functional groups of cations. 1H NMR spectra indicate that there is a dynamic equilibrium between the neutral and ionized species, probably due to the formation of some intermediates in the PILs. The DFT calculations have been carried out to reveal the conformation, and obtain the corresponding IR and 1H NMR spectra of the neutral and ionized species, so that the theoretical support to the experimental results can be provided. The present study will help understand the properties of PILs and provide guidance for further applications of PILs.

 Please wait while we load your content...
Please wait while we load your content...