Solubility and solvation of monosaccharides in ionic liquids†
Abstract
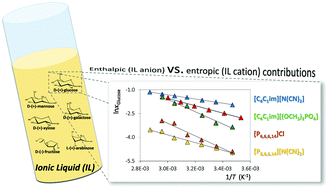
Herein, solubility experimental data for six monosaccharides, viz.D-(+)-glucose, D-(+)-mannose, D-(−)-fructose, D-(+)-galactose, D-(+)-xylose and L-(+)-arabinose, in four ionic liquids (ILs), at temperatures ranging from 288.2 to 348.2 K, were obtained aimed at gathering a better understanding of their solvation ability and molecular-level mechanisms which rule the dissolution process. To ascertain the chemical features that enhance the solubility of monosaccharides, ILs composed of dialkylimidazolium or tetraalkylphosphonium cations combined with the dicyanamide, dimethylphosphate or chloride anions were investigated. It was found that the ranking of the solubility of monosaccharides depends on the IL; yet, D-(+)-xylose is always the most soluble while D-(−)-fructose is the least soluble monosaccharide. The results obtained show that both the IL cation and the anion play a major role in the solubility of monosaccharides. Finally, from the determination of the respective thermodynamic properties of solution, it was found that enthalpic contributions are dominant in the solubilization process. However, the observed differences in the solubilities of monosaccharides in 1-butyl-3-methylimidazolium dicyanamide are ruled by a change in the entropy of solution.


 Please wait while we load your content...
Please wait while we load your content...