The solvation structure of alprazolam†
Abstract
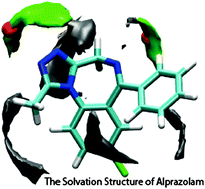
Alprazolam is a benzodiazepine that is commonly prescribed for the treatment of anxiety and other related disorders. Like other benzodiazepines, it is thought to exert its effect through interaction with GABAA receptors. However, it has also been described as a potent and selective protein interaction inhibitor of bromodomain and extra-terminal (BET) proteins. Indeed, the only crystal structure of alprazolam bound to a protein is a complex between alprazolam and the BRD4 bromodomain. The structure shows that the complex also involves many water interactions that mediate contacts between the drug and the protein, a scenario that exists in many drug–protein complexes. How such waters relate to solvation patterns of small molecules may improve our understanding of what dictates their appearance or absence in bridging positions within complexes and thus will be important in terms of future rational drug-design. Here, we use neutron diffraction in conjunction with molecular dynamics simulations to provide a detailed analysis of how water molecules interact with alprazolam in methanol/water mixtures. The agreement between the neutron diffraction and the molecular dynamics is extremely good. We discuss the results in the context of drug design.


 Please wait while we load your content...
Please wait while we load your content...