Irreversible morphological changes of a graphite negative-electrode at high potentials in LiPF6-based electrolyte solution
Abstract
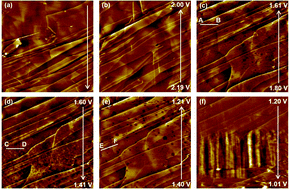
The degradation mechanism of a graphite negative-electrode in LiPF6-based electrolyte solution was investigated using the basal plane of highly oriented pyrolytic graphite (HOPG) as a model electrode. Changes in the surface morphology were observed by in situ atomic force microscopy. In the initial cathodic scan, a number of pits appeared at around 1.75 V vs. Li+/Li, and fine particles formed on the terrace of the HOPG basal plane at about 1.5 V vs. Li+/Li. The fine particles were characterized by spectroscopic analysis, such as X-ray photoelectron spectroscopy and attenuated total reflection Fourier transform infrared spectroscopy. We added one of the components to LiClO4-based electrolyte solution, and successfully reproduced the formation of pits and fine particles on the basal plane of HOPG. Based on these results, the formation mechanisms of pits and fine particle layers were proposed.

 Please wait while we load your content...
Please wait while we load your content...