Tracking dissociation dynamics of strong-field ionized 1,2-dibromoethane with femtosecond XUV transient absorption spectroscopy†
Abstract
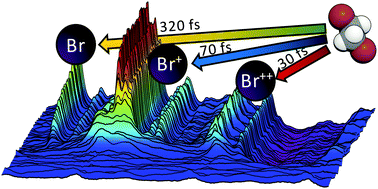
Using femtosecond time-resolved extreme ultraviolet absorption spectroscopy, the dissociation dynamics of the haloalkane 1,2-dibromoethane (DBE) have been explored following strong field ionization by femtosecond near infrared pulses at intensities between 7.5 × 1013 and 2.2 × 1014 W cm−2. The major elimination products are bromine atoms in charge states of 0, +1, and +2. The charge state distribution is strongly dependent on the incident NIR intensity. While the yield of neutral fragments is essentially constant for all measurements, charged fragment yields grow rapidly with increasing NIR intensities with the most pronounced effect observed for Br++. However, the appearance times of all bromine fragments are independent of the incident field strength; these are found to be 320 fs, 70 fs, and 30 fs for Br˙, Br+, and Br++, respectively. Transient molecular ion features assigned to DBE+ and DBE++ are observed, with dynamics linked to the production of Br+ products. Neutral Br˙ atoms are produced on a timescale consistent with dissociation of DBE+ ions on a shallow potential energy surface. The appearance of Br+ ions by dissociative ionization is also seen, as evidenced by the simultaneous decay of a DBE+ ionic species. Dicationic Br++ products emerge within the instrument response time, presumably from Coulomb explosion of triply charged DBE.

 Please wait while we load your content...
Please wait while we load your content...