Legitimate intermediates of oxygen evolution on iridium oxide revealed by in situ electrochemical evanescent wave spectroscopy†
Abstract
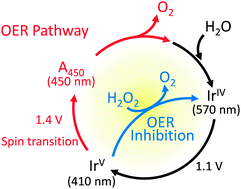
Understanding how the four-electron oxidation of water to dioxygen proceeds in different materials is critical to the rational design of efficient catalysts towards artificial photosynthetic systems. Here, using in situ electrochemical evanescent wave spectroscopy under oxygen-evolving conditions, we report two intermediates of iridium oxide (IrOx), which is the most active and stable catalyst characterized to date in acidic medium. The observed potential dependence of the two intermediates indicated that they were associated with different surface sites, and intermediate scavenging experiments using H2O2 provided insight into their role during catalysis. Notably, an IrV species with an absorption maximum at 450 nm was found to mediate the initial two-electron oxidation of water. Inhibition of the IrV species by H2O2, combined with computational modeling, indicates that the accumulation and concurrent spin-state change of the IrV species is a prerequisite for efficient water oxidation by IrOx electrodes.

 Please wait while we load your content...
Please wait while we load your content...