Synthesis and structure–property relationships of phthalimide and naphthalimide based organic π-conjugated small molecules†
Abstract
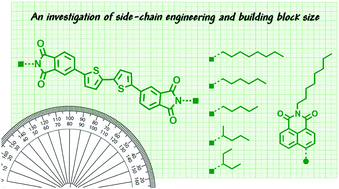
Five organic π-conjugated small molecules with bithiophene-phthalimide backbones bearing alkyl chains of different symmetry, length and branching character were synthesized using optimized microwave and direct heteroarylation protocols. The chosen alkyl chains were 1-ethylpropyl, 1-methylbutyl, pentyl, hexyl and octyl. A sixth compound was also synthesized replacing the phthalimide terminal units with octylnaphthalimide for additional scope. Through the thorough analysis of both thermal and optical properties and the investigation of self-assembly tendencies by single crystal X-ray diffraction and variable angle spectroscopic ellipsometry it is evident that alkyl side chains and building block size influence many facets of material properties. Within this class of materials the 1-ethylpropyl derivative exhibited the most unique behaviour.

 Please wait while we load your content...
Please wait while we load your content...