Changes in the luminescence emission of α,β-unsaturated acrylonitrile derivatives: morphology, polymorphism and solvent effect†
Abstract
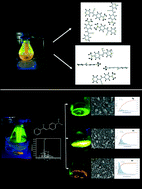
We report an interesting and clear property–structure relationship between the emission wavelength and the morphologies of samples separated by evaporation using a sublimator and a solvent. The compounds are (Z)-3-(4-(dimethylamino)phenyl)-2-(pyridin-4-yl)acrylonitrile Z-DMPyACN (I) and (Z)-3-(4-(diphenylamino)phenyl)-2-(pyridin-2-yl)acrylonitrile Z-DPPyACN (II). I exhibits strong emission in the solid state, but not in a solution, whereas II exhibits emission in a solution and as a solid. Characterization by single-crystal X-ray crystallography showed that the Z-DMPyACN polymorph (Ii) has a monoclinic unit cell with a = 7.0445(2); b = 17.1474(5); c = 10.9776(4) Å, and β = 107.251(4)° and is found in the space group P21/c with Z′ = 1. The orange crystal habit and color are different from the structure reported, (Z′ = 2, block and yellow). The Ii luminescence displayed an absorbance maximum at λmax = 427 nm and an emission maximum at λem = 555 nm. From the Z-DPPyACN II evaporation in the sublimator, three solids were recovered, featuring emission wavelengths that were dependent on the size of the particles (nanoparticles) rather than on the single-crystal structure. The IIg, IIy, and IIo powders showed emission maxima at λem = 546, 558, and 607 nm, respectively. Characterization was carried out by UV-vis, fluorescence and 1H-NMR in different polarity solvents, as well as SCXRD, PXRD and SEM.


 Please wait while we load your content...
Please wait while we load your content...