Testing the limits of synthon engineering: salts of salicylic and sulfosalicylic acid with nucleobases and derivatives†
Abstract
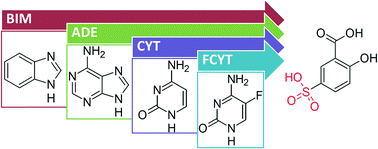
Synthon engineering concepts were tested by using nucleobases and derivatives, namely adenine, cytosine, benzimidazole and fluorocytosine when crystallized with salicylic acid and 5-sulfosalicylic acid. These bases were selected to investigate how the hydrogen bond donor and acceptor groups would influence the crystal structure. Salicylic acid was selected to investigate N–H⋯O hydrogen bonding and synthon formation, while its derivative, 5-sulfosalicylic acid, was selected to see how the sulfonate group would enhance or disrupt these features. Each of the seven complexes obtained was characterized by single crystal X-ray diffraction, which showed that acid–base pairing favored salt formation. All the salicylic acid complexes adopt similar hetero supramolecular synthons, while 5-sulfosalicylic acid forms more complex structures with more than one type of synthon present. Crystal formation using cytosine or fluorocytosine did not result in the predicted synthon formation. This unexpected outcome highlights the current limitations of synthon engineering.

 Please wait while we load your content...
Please wait while we load your content...