Synthesis of hierarchical iron oxide nanostructures from primary nanoparticles and their morphology control via hydrolysis†
Abstract
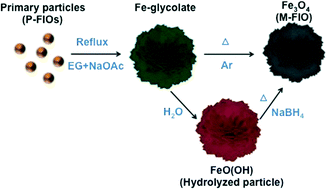
Flower-like hierarchical iron oxide structures have attracted much research attention due to their large surface area, easy separation and re-use, and ability to adsorb toxic ions. However, harsh synthetic conditions such as the use of surfactants and autoclave reactors limit their broader application. Moreover, control over the porosity and morphology of such structures has not yet been investigated. Facile preparation of hierarchical Fe-glycolate particles is possible using pre-prepared (Fe, Ti) oxide nanoparticles without the use of surfactants or autoclave reactors as they act as both precursors and intermediate seeds for self-assembly. The porosity and morphology of the particles are controlled via variable rates of hydrolysis. Replacement of bulky glycolates with smaller water molecules induces surface area and porosity changes in the hierarchical structures. Hierarchical magnetite particles are obtained from the Fe-glycolate and hydrolyzed particles after reductive annealing, and exhibit different surface areas, porosities and magnetic properties. Porosity-controlled magnetites from hydrolyzed particles also show greater adsorption for removing toxic arsenate ions in water.

 Please wait while we load your content...
Please wait while we load your content...