Polymorphism and conformerism in chalcones†
Abstract
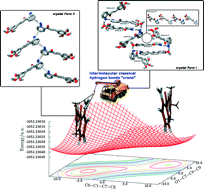
Herein two solid state phenomena have been observed in two chalcones. Firstly, polymorphism has been found in (E)-1-(2-aminophenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (1). This compound crystallizes with only one conformer rather than three in the known reported structure. In the polymorphs, the conformation of the phenyl ring bonded to carbonyl differs slightly. The intermolecular hydrogen bonding from NH2 is the main interaction responsible for polymorphism. Our polymorph is assembled only with weak N–H⋯π interactions instead of strong N–H⋯O and N–H⋯N ones observed in the known structure. DFT calculations reveal that the three conformers of the known polymorph deviate from the minimum energy conformation which is adopted in our crystal form to compensate for the absence of strong intermolecular contacts. Second, conformerism is reported for (E)-1-(3-hydroxyphenyl)-3-(4-nitrophenyl)prop-2-en-1-one (2). Three crystallographically independent molecules are found in the structure of 2: two of them are similarly planar with an anti conformation around the single bond between the carbonyl and α carbons while the third one is twisted, with its phenyl ring bonded to carbonyl rotated by ca. 60° besides presenting a syn conformation around the corresponding rotatable bond. Both conformational features are related to the crystal packing, allowing accommodation of twisted molecules onto the layers made up of hydrogen bonded planar molecules. Furthermore, our potential energy surface scans indicate that the planar and twisted conformations of the phenyl ring bonded to carbonyl are not compatible with the syn and anti conformations of the chalcone skeleton.

 Please wait while we load your content...
Please wait while we load your content...