A new strategy towards the synthesis of a room-temperature discotic nematic liquid crystal employing triphenylene and pentaalkynylbenzene units†
Abstract
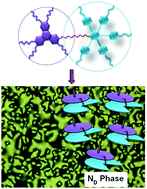
A new approach is reported for the design of a room-temperature discotic nematic (ND) liquid crystal (LC) dimer consisting of a triphenylene and a pentaalkynylbenzene unit linked via flexible alkyl spacers. The formation of the ND phase is realized most likely through folding of the dimeric molecule that prevent stacking between the triphenylene units, as suggested by modelling in the mesophase derived from X-ray scattering results and high-level DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...