Regio- and stereoselective β-mannosylation using a boronic acid catalyst and its application in the synthesis of a tetrasaccharide repeating unit of lipopolysaccharide derived from E. coli O75†
Abstract
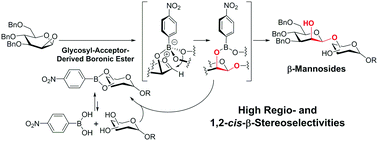
Regio- and stereoselective β-mannosylations using 1,2-anhydromannose and diol sugar acceptors in the presence of a boronic acid catalyst proceeded smoothly to give the corresponding β-mannosides with high regio- and β-stereoselectivities in high yields without further additives under mild conditions. In addition, this glycosylation method was applied successfully to the synthesis of a tetrasaccharide repeating unit of lipopolysaccharide (LPS) derived from E. coli O75.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...