J-aggregation of a sulfur-substituted naphthalenediimide (NDI) with remarkably bright fluorescence†
Abstract
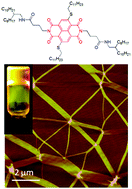
This communication reveals the H-bonding driven supramolecular assembly of a sulfur-substituted naphthalenediimide leading to the formation of very strong (Tg > 90 °C) organogel in aliphatic hydrocarbons. Mechanistic investigation reveals nucleation–elongation pathway for self-assembly. Photophysical studies show explicit features of classical J-aggregation which reduces the non-radiative fluorescence rate constant considerably and thus results in a remarkable fluorescence enhancement (ΦPL increases from 1% to 30%) which is unprecedented in the entire NDI literature.

 Please wait while we load your content...
Please wait while we load your content...