α-Methylation follows condensation in the gephyronic acid modular polyketide synthase†
Abstract
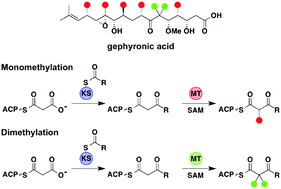
C-methyltransferases (MTs) from modular polyketide synthase assembly lines are relatively rare and unexplored domains that are responsible for installing α-methyl groups into nascent polyketide backbones. The stage at which these synthase-embedded enzymes operate during polyketide biosynthesis has yet to be conclusively demonstrated. In this work we establish the activity and substrate preference for six MTs from the gephyronic acid polyketide synthase and demonstrate their ability to methylate both N-acetylcysteamine- and acyl carrier protein-linked β-ketoacylthioester substrates but not malonyl thioester equivalents. These data strongly indicate that MT-catalyzed methylation occurs immediately downstream of ketosynthase-mediated condensation during polyketide assembly. This work represents the first successful report of MT-catalyzed mono- and dimethylation of simple thioester substrates and provides the groundwork for future mechanistic and engineering studies on this important but poorly understood enzymatic domain.

 Please wait while we load your content...
Please wait while we load your content...