Preparation of difluoromethylthioethers through difluoromethylation of disulfides using TMS-CF2H†
Abstract
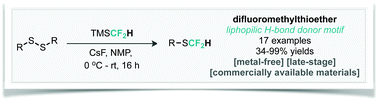
We report an operationally simple, metal-free approach for the late-stage introduction of the important lipophilic hydrogen-bond donor motif, SCF2H. This reaction converts diaryl- and dialkyl-disulfides into the corresponding aryl/alkyl–SCF2H through the nucleophilic transfer of a difluoromethyl group with good functional group tolerance. This method is notable for its use of commercially available TMSCF2H, and does not rely on the need for handling of sensitive metal complexes.

 Please wait while we load your content...
Please wait while we load your content...