Highly photoluminescent copper carbene complexes based on prompt rather than delayed fluorescence†
Abstract
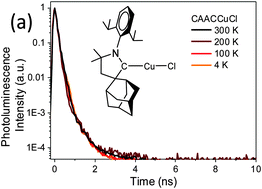
Linear two-coordinate copper complexes of cyclic (alkyl)(amino)carbenes (CAAC)CuX (X = halide) show photoluminescence with solid-state quantum yields of up to 96%; in contrast to previously reported Cu photoemitters the emission is independent of temperature over the range T = 4–300 K and occurs very efficiently by prompt rather than delayed fluorescence, with lifetimes in the sub-nanosecond range.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...