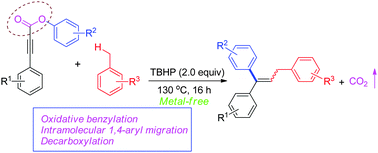
Direct difunctionalization of activated alkynes via domino oxidative benzylation/1,4-aryl migration/decarboxylation reactions under metal-free conditions†
Abstract
The oxidative difunctionalization of aryl alkynoates via benzyl radical initiated intramolecular 1,4-aryl migration and decarboxylation tandem process was developed in the presence of tert-butyl hydroperoxide as an oxidant, which provides a direct and selective route to a variety of trisubstituted alkenes from simple toluene derivatives and aryl alkynoates.

 Please wait while we load your content...
Please wait while we load your content...