Room temperature methoxylation in zeolites: insight into a key step of the methanol-to-hydrocarbons process†
Abstract
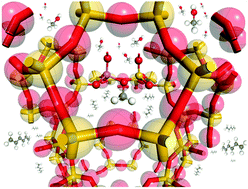
Neutron scattering methods observed complete room temperature conversion of methanol to framework methoxy in a commercial sample of methanol-to-hydrocarbons (MTH) catalyst H-ZSM-5, evidenced by methanol immobility and vibrational spectra matched by ab initio calculations. No methoxylation was observed in a commercial HY sample, attributed to the dealumination involved in high silica HY synthesis.
- This article is part of the themed collection: Commemorating Michael Faraday (1791-1867)


 Please wait while we load your content...
Please wait while we load your content...