A FRET-based biosensor for the detection of neutrophil elastase†
Abstract
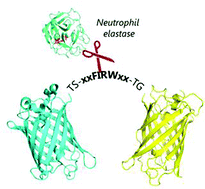
The direct and specific detection of biomarkers is crucial as it can allow monitoring the state of a tissue or wound as well as the progression of the inflammatory process. Neutrophil elastase (NE) plays an important role in many biological processes. It is involved in inflammatory diseases and is enriched in inflamed tissues, in wound exudate, and in the sputum of cystic fibrosis patients. In order to detect NE, we designed a NE-specific protein sensor whose fluorescence features are altered upon selective cleavage by NE at physiological concentration. The biosensor consists of two fluorescent GFP-derived proteins connected by a short peptide linker containing a NE-specific recognition sequence. Due to the close proximity of the two fluorescent proteins, Förster resonance energy transfer (FRET) occurs in the uncleaved form and, upon cleavage by NE, a decrease of FRET signal is observed. In this study, the construct design, the influence of the linker length, as well as the specificity for NE are described. Furthermore, the influence of biosensor immobilization on the functionality is analysed. By engineering the recognition sequence of the linker, the presented system can potentially be easily adapted to detect other proteases such as cathepsin, caspases or matrix metalloproteases.


 Please wait while we load your content...
Please wait while we load your content...