Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting†
Abstract
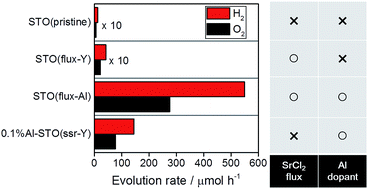
SrTiO3 is a photocatalyst that is well known for its activity for the overall water splitting reaction under UV light irradiation. In this study, the effects of SrCl2 flux treatments and Al doping on the photocatalytic properties of SrTiO3 were investigated. The SrTiO3, which showed an apparent quantum efficiency of 30% at 360 nm in the overall water splitting reaction, the highest value reported so far, was prepared by SrCl2 flux treatments in alumina crucibles. Scanning electron microscopy and X-ray diffractometry revealed that the flux-treated SrTiO3 consisted of well-crystalline particles with a cubic shape reflecting the perovskite-type structure. Inductively coupled plasma optical emission spectroscopy revealed that Al ions from the alumina crucibles were incorporated into the SrTiO3 samples. The SrTiO3 that was treated with SrCl2 flux in Al-free conditions showed a marginal improvement in photocatalytic activity despite the high crystallinity and the clear crystal habit. Doping SrTiO3 with Al improved the photocatalytic activity even without SrCl2 treatment. These results suggested that Al doping was a principal factor in the dramatic improvement in the water splitting activity of the flux-treated SrTiO3. The effects of flux treatments and Al doping on the morphology and water splitting activity of SrTiO3 were discussed separately.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...