Gold(iii) tetraarylporphyrin amino acid derivatives: ligand or metal centred redox chemistry?†
Abstract
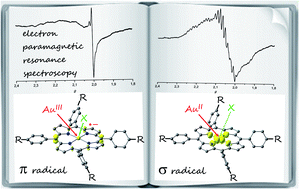
Meso tetraarylporphyrinato gold(III) cations bearing different substituents at the aryl substituents (COOMe, COOH, NO2, NH2, NHAc, H, OnBu, CF3) were prepared and characterised. Their reversible one-electron reductions were studied by (spectro)electrochemical means as well as by selective chemical one-electron reduction using cobaltocene. The preferred location of the spin density, namely gold centred or porphyrin centred, was probed by electron paramagnetic resonance spectroscopy (g values, 197Au hyperfine coupling) as well as by density functional theory calculations (spin densities). In all cases studied experimentally and theoretically, the gold(II) valence isomer (5d9 electron configuration) is preferred over the porphyrin π radical anion. In the hexafluorophosphate salt of the nitro derivative a further nitro π radical anion valence isomeric species is significantly populated. In the presence of chloride ions this nitro π radical anion/AuII valence isomeric equilibrium evolves towards the porphyrin π radical anion. The electronic structures of the nitro π radical and the AuII σ radical valence isomers (5dx2−y2 orbital) could be calculated by DFT methods. The electron transfer pathway between the nitro π radical anion and the AuII valence isomer is well described by the location of the hexfluorophosphate counterion, the Au–N distances (corresponding to the totally symmetric stretching vibration), the symmetric stretching mode of the NO2 substituent and a meso-nitrophenyl rotation. The specific geometric and electronic properties of the favoured gold(II) σ radical valence isomer, namely counterion dislocation and σ symmetry of the redox orbital, might stabilise charge-shifted states [(gold(II) porphyrin)-donor˙+] by retarding the back electron transfer to give the ground state (gold(III) porphyrin)-donor. This will guide the design of (photo-induced) electron transfer pathways with tetraarylporphyrinato gold(III) complexes as electron acceptors.

 Please wait while we load your content...
Please wait while we load your content...