Near-IR absorbing donor–acceptor ligand-to-ligand charge-transfer complexes of nickel(ii)†
Abstract
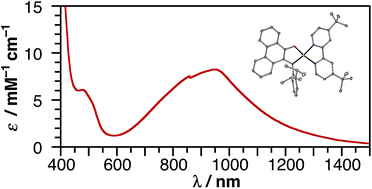
A new series of square-planar nickel(II) donor–acceptor complexes exhibiting ligand-to-ligand charge-transfer (LL'CT) transitions have been prepared. Whereas the use of a catecholate donor ligand in conjunction with a bipyridyl acceptor ligand affords a complex that absorbs throughout the visible region, the use of a azanidophenolate donor ligands in conjunction with a bipyridyl acceptor ligand affords complexes that absorbs well into the near-IR region of the solar spectrum. Three new complexes, (cat)Ni(bpytBu2) (1; (cat)2− = 3,5-di-tert-butyl-1,2-catecholate; bpytBu2 = 4,4′-di-tert-butyl-2,2′-bipyridine), (ap)Ni(bpytBu2) (2; (ap)2− = 4,6-di-tert-butyl-2-(2,6-diisopropylphenylazanido)phenolate), and (apPh)Ni(bpytBu2) (3; (apPh)2− = 10-(2,6-diisopropylphenylazanido)-9-phenanthrolate), have been prepared and characterized by structural, electrochemical, and spectroscopic methods. Whereas all three square-planar complexes show multiple reversible one-electron redox-processes and strong LL'CT absorption bands, in azanidophenolate complexes 2 and 3, the LL'CT absorption covers the near-IR region from 700–1200 nm. The electronic absorption spectra and ground state electrochemical data for 2 and 3 provide an estimate of their excited-state reduction potentials, E+/*, of −1.3 V vs. SCE, making them as potent as the singlet excited state of [Ru(bpy)3]2+.

 Please wait while we load your content...
Please wait while we load your content...