Metal-free radical thiolations mediated by very weak bases†
Abstract
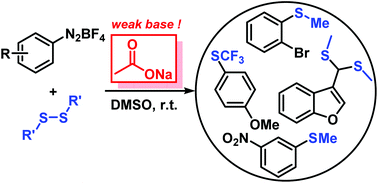
Aromatic thioethers and analogous heavier chalcogenides were prepared by reaction of arene-diazonium salts with disulfides in the presence of the cheap and weak base NaOAc. The mild and practical reaction conditions (equimolar reagents, DMSO, r.t., 8 h) tolerate various functional groups (e.g. Br, Cl, NO2, CO2R, OH, SCF3, furans). Mechanistic studies indicate the operation of a radical aromatic substitution mechanism via aryl, acetyloxyl, thiyl, and dimsyl radicals.

 Please wait while we load your content...
Please wait while we load your content...