Synthesis of α-methylene-δ-oxo-γ-amino esters via Rh(ii)-catalyzed coupling of 1-sulfonyl-1,2,3-triazoles with Morita–Baylis–Hillman adducts†
Abstract
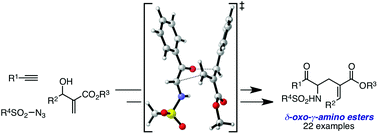
A rhodium(II)-catalyzed coupling of 1-sulfonyl-1,2,3-triazoles, prepared from 1-alkynes and sulfonyl azides, with Morita–Baylis–Hillman (MBH) adducts afforded highly functionalized α-methylene-δ-oxo-γ-amino esters in excellent yields with broad functional group tolerance. This transformation can also be successfully accomplished as a multicomponent all-in-one-pot reaction of 1-alkynes, sulfonyl azides and MBH adducts in the presence of Cu(I) and Rh(II) catalysts.

 Please wait while we load your content...
Please wait while we load your content...