Toward solid-phase peptide fragment ligation by a traceless-Ugi multicomponent reaction approach†
Abstract
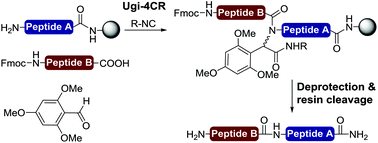
A new methodology to couple peptide fragments on solid support using a traceless isocyanide-based multicomponent reaction is described. The approach uses a microwave-assisted on-resin Ugi four-component reaction to attach a carboxyl free peptide to a supported peptide bearing a free N-terminal amine via the formation of an N-protected amide bond at the ligation site. Afterward, the generated backbone amide protecting group can be efficiently removed by microwave-assisted acidolysis with trifluoroacetic acid to afford a fully deprotected peptide. This straightforward Ugi reaction/deprotection approach was applied to condense various fragment lengths and provided a variety of oligopeptides.

 Please wait while we load your content...
Please wait while we load your content...