Total synthesis of cyclomarins A, C and D, marine cyclic peptides with interesting anti-tuberculosis and anti-malaria activities†
Abstract
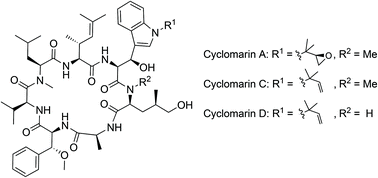
Cyclomarins are cyclic heptapeptides containing four unusual amino acids. New synthetic protocols toward their synthesis have been developed, leading to the synthesis and biological evaluation of three natural occurring cyclomarins. Interestingly, cyclomarins address two completely different targets: Clp C1, a subunit of the caseinolytic protease of Mycobacterium tuberculosis (MTB), as well as PfAp3Ase of Plasmodium falciparum. Therefore, cyclomarins are interesting lead structures for the development of drugs against tuberculosis and malaria.

 Please wait while we load your content...
Please wait while we load your content...