Asymmetric aza-Morita–Baylis–Hillman reactions of chiral N-phosphonyl imines with acrylates via GAP chemistry/technology†
Abstract
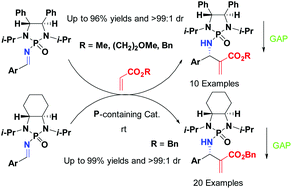
Chiral N-phosphonyl imines have been proven to be efficient electrophilic acceptors for asymmetric aza-Morita–Baylis–Hillman (aza-MBH) reactions with acrylates under convenient conditions. Thirty examples of β-amino acrylates were generated in high yields (up to 99.4%) and diastereoselectivity (up to >99 : 1 dr) in an atom-economical fashion. The synthesis was proved to follow the GAP (group-assisted purification) chemistry, i.e., the pure products can be obtained simply by washing the crude products with hexane/ethyl acetate (v/v, 10/1) without the use of chromatography or recrystallization. DFT calculations were also conducted to support an asymmetric induction model accounting for high diastereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...