Micellization of alkyltrimethylammonium bromide surfactants in choline chloride:glycerol deep eutectic solvent†
Abstract
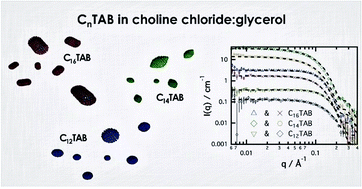
Deep eutectic solvents have shown the ability to promote the self-assembly of surfactants in solution. However, some differences have been found compared with self-assembly in pure water and other polar organic solvents. The behaviour of alkyltrimethylammonium bromides in choline chloride:glycerol deep eutectic solvent has been studied by means of surface tension, X-ray and neutron reflectivity and small-angle neutron scattering. The surfactants were found to remain surface active and showed comparable critical micelle concentrations to the same surfactants in water. Our scattering studies demonstrate that these surfactants form globular micelles with ellipsoidal shape in solution. The size, shape and aggregation number of the aggregates were found to vary with the chain length of the surfactant. Specific solvent-headgroup interactions were not found in this system, unlike those we have previously postulated for anionic surfactants in choline chloride deep eutectic solvents.


 Please wait while we load your content...
Please wait while we load your content...