Direct inference of site strength in basic solids upon CO2 adsorption: enthalpy–entropy compensation effects†
Abstract
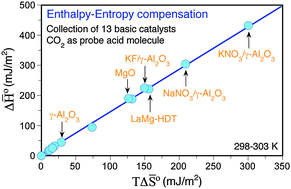
The adsorption of CO2 coupled to calorimetry is a state-of-the-art technique for characterizing the basic properties of solids. In this paper, we show that the differential heat and entropy curves measured upon CO2 adsorption on a basic solid can be reasonably estimated from a single CO2 isotherm with no need for any independent heat (calorimetric) measurement. Our method relies on two important observations: (1) formulation of generalized F–H–TS thermodynamic isotherms, the former (F) being directly generated from the raw CO2 isotherms, and (2) the presence of unexpected enthalpy–entropy compensation effects upon CO2 adsorption linking the integral enthalpy and entropy of adsorption until saturation for different solids. Our thermodynamic method has been validated using a broad library of basic solids with variable site strength and heterogeneity. Finally, a new scale of basicity is proposed using the parameters fitted from the thermodynamic isotherm (free energy basis) as descriptors of basic strength. This method opens an avenue to the inference of site strength of basic solids without the need for expensive calorimeters.

 Please wait while we load your content...
Please wait while we load your content...