An artificial photosynthetic model based on a molecular triad of boron dipyrromethene and phthalocyanine†
Abstract
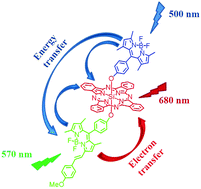
A boron dipyrromethene (BDP) unit and its monostyryl derivative (MSBDP) were introduced at the axial positions of a silicon(IV) phthalocyanine (SiPc) core. The absorption spectrum of this compound virtually covered the entire visible region (300–700 nm) and could be interpreted as a superposition of the spectra of individual components. The intramolecular photoinduced energy and charge transfer processes of this triad were studied using steady-state and time-resolved spectroscopic methods in polar and nonpolar solvents. Upon BDP-part excitation, a fast and highly efficient excitation energy transfer (EET) occurred resulting in strong quenching of its fluorescence and the formation of the first excited singlet state of SiPc or MSBDP. It was found that both EET and charge transfer (CT) processes competed with each other in the depopulation of the first excited singlet state of the MSBDP moiety. The former strongly superseded CT in nonpolar toluene, whereas the latter was dominant in a polar environment. Direct or indirect (via EET) excitation of the SiPc-part of the triad was followed by CT yielding the charge-separated (CS) species BDP–SiPc˙−–MSBDP˙+. The energy gap between the CS state and the S1-state of the SiPc moiety was found to be only 0.06 eV in toluene, which facilitated the back CT process and resulted in the appearance of thermally activated delayed fluorescence. With increasing solvent polarity, the energy of the CS state reduced resulting in the disappearance of the delayed fluorescence in CHCl3, tetrahydrofuran or N,N-dimethylformamide. The charge recombination rate, kCR, was very fast in polar DMF (3.3 × 1010 s−1), whereas this process was two-orders of magnitude slower in nonpolar toluene (kCR = 4.0 × 108 s−1).

 Please wait while we load your content...
Please wait while we load your content...