Liposome chaperon in cell-free membrane protein synthesis: one-step preparation of KcsA-integrated liposomes and electrophysiological analysis by the planar bilayer method
Abstract
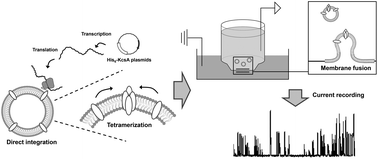
Chaperoning functions of liposomes were investigated using cell-free membrane protein synthesis. KcsA potassium channel-reconstituted liposomes were prepared directly using cell-free protein synthesis. In the absence of liposomes, all synthesized KcsA protein aggregated. In the presence of liposomes, however, synthesized KcsA spontaneously integrated into the liposome membrane. The KscA-reconstituted liposomes were transferred to the planar bilayer across a small hole in a thin plastic sheet and the channel function of KcsA was examined. The original electrophysiological activities, such as voltage- and pH-dependence, were observed. These results suggested that in cell-free membrane protein synthesis, liposomes act as chaperones, preventing aggregation and assisting in folding and tetrameric formation, thereby allowing full channel activity.

 Please wait while we load your content...
Please wait while we load your content...