Self-assembly of tetra(aniline) nanowires in acidic aqueous media with ultrasonic irradiation†
Abstract
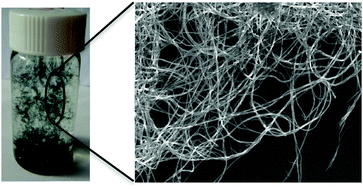
An environmentally friendly method is developed to explore the self-assembly of Ph/NH2-capped tetra(aniline), TANI, nanowires in acidic aqueous media with ultrasonic irradiation. Ultrasonic irradiation is demonstrated to be an effective method to achieve self-assembled thermodynamic equilibrium for nanostructure formation in only 2 minutes. Further assembly, i.e., the formation of thicker TANI nanowires in acidic solution left undisturbed for 96 h without the addition of any organic solvent, is also investigated. The self-assembly behaviour of TANI is studied using FT-IR, Raman, UV-Vis spectroscopy, thermogravimetric analysis, X-ray diffraction, and scanning electron microscopy. Investigations suggest that extra hydrogen bonding associated with the protonation, electrostatic interactions and π–π stacking interaction are important for the self-organization of TANI nanowires. Furthermore, the assembly behaviour of TANI nanowires is dependent on the properties of the dopant, including size and concentration, and reflected in the conductivity of the assembled structures. These results provide insight to understand and tune the self-assembly behaviour of nanostructured oligo(aniline)s in complex dopant-containing systems, and form the basis for further detailed mechanistic studies.

 Please wait while we load your content...
Please wait while we load your content...