Molecular cobalt–salen complexes as novel cocatalysts for highly efficient photocatalytic hydrogen production over a CdS nanorod photosensitizer under visible light†
Abstract
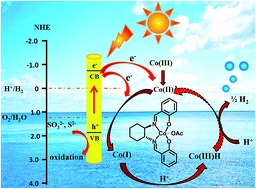
An efficient photocatalytic system is highly demanded for the production of hydrogen fuel through water splitting. Herein, we report an artificial photocatalytic system made of low-cost materials for high-performance H2 production from water. The new system contains semiconductors (CdS nanorods) as the photosensitizer, a cobalt–salen complex as the H2 evolution cocatalyst, and Na2S and Na2SO3 as sacrificial electron donors. Under optimal conditions, the highest hydrogen evolution turnover number reached 64 700 after 37 hours and the rate was 106 μmol h−1 mg−1, which is much higher than when using CdS NRs and also is among the best for photocatalytic systems using molecular cocatalysts for H2 production. The highest apparent quantum yield (AQY) was ∼29% at 420 nm. Steady state photoluminescence (PL) spectra and time-resolved photoluminescence (TRPL) decay spectra revealed that the system allows effective electron transfer from the excited CdS NRs to the cobalt–salen complex for highly efficient H2 production.

 Please wait while we load your content...
Please wait while we load your content...