A theory for the phase behavior of mixtures of active particles†
Abstract
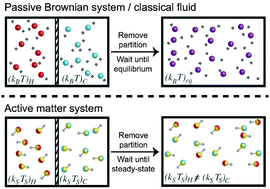
Systems at equilibrium like molecular or colloidal suspensions have a well-defined thermal energy kBT that quantifies the particles' kinetic energy and gauges how “hot” or “cold” the system is. For systems far from equilibrium, such as active matter, it is unclear whether the concept of a “temperature” exists and whether self-propelled entities are capable of thermally equilibrating like passive Brownian suspensions. Here we develop a simple mechanical theory to study the phase behavior and “temperature” of a mixture of self-propelled particles. A mixture of active swimmers and passive Brownian particles is an ideal system for discovery of the temperature of active matter and the quantities that get shared upon particle collisions. We derive an explicit equation of state for the active/passive mixture to compute a phase diagram and to generalize thermodynamic concepts like the chemical potential and free energy for a mixture of nonequilibrium species. We find that different stability criteria predict in general different phase boundaries, facilitating considerations in simulations and experiments about which ensemble of variables are held fixed and varied.

 Please wait while we load your content...
Please wait while we load your content...