Diverse reactivity of a tricoordinate organoboron L2PhB: (L = oxazol-2-ylidene) towards alkali metal, group 9 metal, and coinage metal precursors†
Abstract
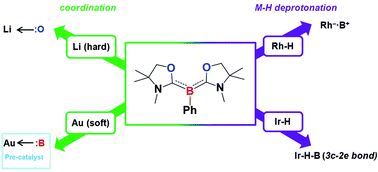
The reactivity of a tricoordinate organoboron L2PhB: (L = oxazol-2-ylidene) 1 towards metal precursors and its coordination chemistry were comprehensively studied. While the boron center in 1 is reluctant to coordinate to the alkali metals in their trifluoromethanesulfonate salts (MOTf) (M = Li, Na, K), the unprecedented compound 2 containing two L2PhB: units linked by a cyclic Li(OTf)2Li spacer was obtained from the reaction of 1 with LiOTf. Treatment of 1 with group 9 metal complexes [MCl(COD)]2 (M = Rh, Ir) afforded the first zwitterionic rhodium(I)–boronium complex 3 and the iridium(III)–borane complex 4, respectively. The reaction pathway may involve C–H activation followed by proton migration from the metals to the boron center, demonstrating the first example of the deprotonation of metal hydrides by a basic boron. In the reactions with coinage metals, 1 could act as a two-electron reducing agent towards the metal chlorides MCl (M = Cu, Ag, Au). Meanwhile, the reaction of 1 with gold chloride supported by a N-heterocyclic carbene (NHC) produced a heteroleptic cationic gold complex [(L2PhB)Au(NHC)]Cl (6) featuring both carbene and L2PhB: ligands on the gold atom. In contrast, an isolable gold chloride complex (L2PhB)AuCl (8) was obtained by direct complexation between 1 and triphenylphosphine-gold chloride via ligand exchange. X-ray diffraction analysis and computational studies revealed the nature of the B:→Au bonding interaction in complexes 6 and 8. Natural Population Analysis (NPA) and Natural Bond Orbital (NBO) analysis support the strong σ-donating property of the L2PhB: ligand. Moreover, preliminary studies showed that complex 8 can serve as an efficient precatalyst for the addition of X–H (X = N, O, C) to alkynes under ambient conditions, demonstrating the first application of a metal complex featuring a neutral boron-based ligand in catalysis.

 Please wait while we load your content...
Please wait while we load your content...