Site-specific bioalkylation of rapamycin by the RapM 16-O-methyltransferase†
Abstract
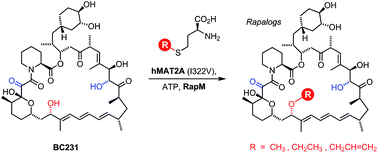
The methylation of natural products by S-adenosyl methionine (AdoMet, also known as SAM)-dependent methyltransferase enzymes is a common tailoring step in many biosynthetic pathways. The introduction of methyl substituents can affect the biological and physicochemical properties of the secondary metabolites produced. Recently it has become apparent that some AdoMet-dependent methyltransferases exhibit promiscuity and will accept AdoMet analogues enabling the transfer of alternative alkyl groups. In this study we have characterised a methyltransferase, RapM, which is involved in the biosynthesis of the potent immunosuppressive agent rapamycin. We have shown that recombinant RapM regioselectively methylates the C16 hydroxyl group of desmethyl rapamycin precursors in vitro and is promiscuous in accepting alternative co-factors in addition to AdoMet. A coupled enzyme system was developed, including a mutant human enzyme methionine adenosyl transferase (MAT), along with RapM, which was used to prepare alkylated rapamycin derivatives (rapalogs) with alternative ethyl and allyl ether groups, derived from simple S-ethyl or S-allyl methionine analogues. There are two other methyltransferases RapI and RapQ which provide methyl substituents of rapamycin. Consequently, using the enzymatic approach described here, it should be possible to generate a diverse array of alkylated rapalogs, with altered properties, that would be difficult to obtain by traditional synthetic approaches.

 Please wait while we load your content...
Please wait while we load your content...