The positive effect of anatase and rutile on the brookite-photocatalyzed degradation of phenol†
Abstract
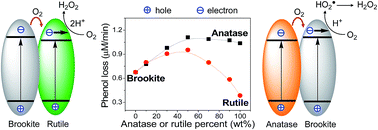
In this study, the biphase effect of brookite with anatase or rutile has been reinvestigated by using phenol degradation in aqueous solution as a model reaction. Prior to use, each oxide was sintered at 500 °C. Then two oxides were mixed in alcohol, followed by drying and sintering at 450 °C. Solid characterization showed that each oxide remained intact, in terms of the average crystallite size and surface area. As the weight percent of anatase or rutile in the mixture increased, the apparent activity of TiO2 for phenol degradation under O2 increased, and then decreased. A maximum apparent activity of TiO2 was observed at 50% of anatase and rutile, respectively. However, with the same amount of silver ions on the oxide surface for phenol degradation under N2, the intrinsic activity of TiO2 became independent of the content of either anatase or rutile. These observations indicate that the thermodynamically possible charge transfer between two phases does not occur in practice. Moreover, for the photocatalytic reduction of O2 to H2O2 in an aqueous suspension containing excess phenol, anatase was more active than brookite, followed by rutile. Accordingly, it is proposed that there is an interfacial O2 transfer from anatase to brookite, and/or from brookite to rutile. This would improve the efficiency of the charge separation and consequently increase the rate of phenol degradation at interfaces.

 Please wait while we load your content...
Please wait while we load your content...