Hydrogel formed by the co-assembly of sodium laurate and silica nanoparticles†
Abstract
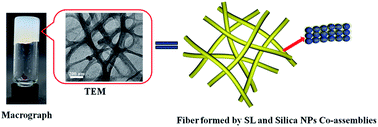
Co-assembly between surfactants and inorganic nanoparticles is an appealing research field which has been proved to be an effective approach to create hybrid materials. In this paper, a new type of co-assembled hydrogel formed by an anionic surfactant and nanoparticles is reported. The hydrogel can be achieved by mixing the anionic surfactant, sodium laurate (SL), with silica nanoparticles (silica NPs) in an aqueous solution with dissolved potassium chloride (KCl). It was found that the silica NPs and SL co-assembled to intertwined 1D fibers as well as a macroscopic hydrogel, where the complexes of amphiphilic molecules and nanoparticles acted as building blocks. We proved that the formation of the hydrogels originates from the hydrogen bond and hydrophobic effect in the SL and silica NPs co-assembled system. In addition, the silica NPs assembled with SL molecules by hydrogen bonds, rather than SL aggregates, which is different with the conventional studies. The novel phenomenon of co-assembly between a surfactant and nanoparticles not only provides a new strategy for the construction of co-assemblies, but also may advance a better understanding of the fundamental science.

 Please wait while we load your content...
Please wait while we load your content...