Solvation thermodynamics: two formulations and some misunderstandings
Abstract
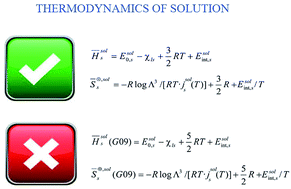
Conventional (Fowler–Guggenheim) and Ben-Naim's formulations of solvation thermodynamics are analyzed in parallel, emphasizing their differences and stressing their interconnections. The pivotal equations relating the thermodynamic functions in both theories are derived. Connections with Pierotti–Abraham's cavity-interaction partition model are also contemplated in detail. Evidence is presented that misinterpretations of some of the derived equations lead to wrong estimates of solvation thermodynamic quantities that have been detected in the output of some of the most popular quantum chemistry software packages (Gaussian 09).

 Please wait while we load your content...
Please wait while we load your content...