Labile dioxy-functionalised zwitterionic imidazolinium salt: access to zwitterionic and neutral imidazolidin-2-ylidene derivatives and π-acceptor properties of imidazolidine-2-selones†
Abstract
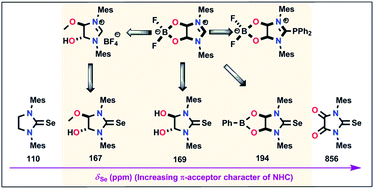
The dioxy-functionalised zwitterionic imidazolinium salt (3) is synthesised by the condensation of N,N′-dimesitylformamidine with glyoxal in the presence of ammonium tetrafluoroborate. The nucleophilicity of the carbene carbon is ascertained using the in situ generated anionic NHC from 3 with different electrophiles. Furthermore, 3 consists of a labile anionic five membered C2O2B at the backbone position of the imidazolinium unit, which can be tailored to prepare neutral NHC chalcogen compounds containing symmetrical and unsymmetrical oxy-functionalisation at the backbone. Furthermore, the π-acceptor properties of NHCs were evaluated using 77Se NMR in their respective imidazolidine-2-selones.

 Please wait while we load your content...
Please wait while we load your content...